iPSC Core facility Nantes - induced pluripotent stem cells
Who we are
The iPSC platform specialises in the culture of human pluripotent stem cells. It is dedicated in particular to the reprogramming of adult cells into induced pluripotent stem cells (iPSCs), as well as to the genome editing of these cells or control iPSC lines.
iPSC cells have the unique ability to differentiate into any cell type in the adult organism. This property makes them a tool of choice for regenerative medicine, the development of new treatments, pharmaceutical research, and the study of genetic disease mechanisms.
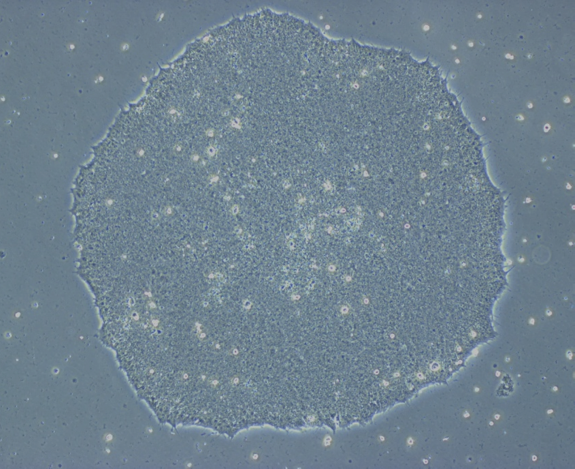
iPSC ©plateforme iPSC
Created in 2012, the iPSC Platform has produced more than 250 iPSC lines for 80 research teams.
Since genome editing became available in 2024, 15 iPSC lines have been edited.
In partnership with Nantes University Hospital, the iPSC platform has developed, characterised and made available to the scientific community a line of human embryonic stem cells (hESCs).
As part of the European (CoReuStem) and international (Coredinates) networks of pluripotent stem cell platforms, the iPSC platform shares its expertise through training courses offered locally and nationally.
Our expertises
- Reprogrammable primary cells: Fibroblasts, PBMCs, amniotic cells, urinary cells
- Use of non-integrative techniques: Sendai virus or mRNA
- Quality controls performed on cell lines: qPCR of pluripotency genes, verification of absence of SeV transgene (where applicable), verification of genomic integrity, validation of cell differentiation capacity, absence of mycoplasma.

Reprogramming ©NU
The platform also advises teams on managing their own cell banks, particularly for setting up Master and Working Cell Banks.
- in Nantes, with the infrastructure ION
- in Western France, through the OuestOÏD federating project

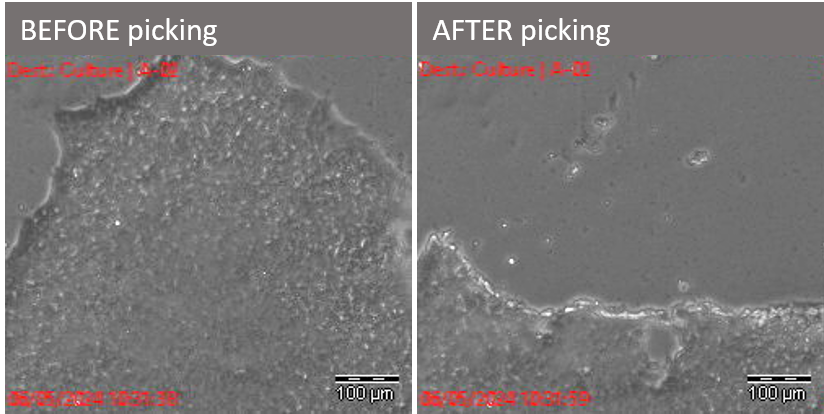
iPSC picked with the Cell Celector ©iPSC core facility
This training course aims to provide theoretical (9 hours of lectures) and practical (13 hours of practical work) foundations in the culture of pluripotent stem cells.
Each trainee receives personalised support in setting up this culture in their laboratory and in developing their research project.
Notable Publications
- Martin L, Maric D, Idriss S, et al. Identification of Hepatic-like EPO as a Cause of Polycythemia. N Engl J Med. 2025;392(17):1684-1697. doi:10.1056/NEJMoa241495
- Geryk M, Canac R, Forest V, et al. Generation of a patient-specific induced pluripotent stem cell line carrying the DES p.R406W mutation, an isogenic control and a DES p.R406W knock-in line. Stem Cell Res. 2024;77:103396. doi:10.1016/j.scr.2024.103396
- Warin J, Vedrenne N, Tam V, et al. In vitro and in vivo models define a molecular signature reference for human embryonic notochordal cells. iScience. 2024;27(2):109018. Published 2024 Jan 26. doi:10.1016/j.isci.2024.109018
- Delamare M, Le Roy A, Pacault M, et al. Characterization of genetic variants in the EGLN1/PHD2 gene identified in a European collection of patients with erythrocytosis. Haematologica. 2023;108(11):3068-3085. Published 2023 Nov 1. doi:10.3324/haematol.2023.282913
- Girardeau A, Atticus D, Canac R, et al. Generation of human induced pluripotent stem cell lines from four unrelated healthy control donors carrying European genetic background. Stem Cell Res. 2022;59:102647. doi:10.1016/j.scr.2021.102647
- Al Sayed ZR, Jouni M, Gourraud JB, et al. A consistent arrhythmogenic trait in Brugada syndrome cellular phenotype. Clin Transl Med. 2021;11(6):e413. doi:10.1002/ctm2.413
- Castel G, Meistermann D, Bretin B, et al. Induction of Human Trophoblast Stem Cells from Somatic Cells and Pluripotent Stem Cells. Cell Rep. 2020;33(8):108419. doi:10.1016/j.celrep.2020.108419
- Al Sayed ZR, Canac R, Cimarosti B, et al. Human model of IRX5 mutations reveals key role for this transcription factor in ventricular conduction. Cardiovasc Res. 2021;117(9):2092-2107. doi:10.1093/cvr/cvaa259
- Montibus B, Cercy J, Bouschet T, et al. TET3 controls the expression of the H3K27me3 demethylase Kdm6b during neural commitment. Cell Mol Life Sci. 2021;78(2):757-768. doi:10.1007/s00018-020-03541-8
- Colombier P, Halgand B, Chédeville C, et al. NOTO Transcription Factor Directs Human Induced Pluripotent Stem Cell-Derived Mesendoderm Progenitors to a Notochordal Fate. Cells. 2020;9(2):509. Published 2020 Feb 24. doi:10.3390/cells9020509
- Cogné B, Bouameur JE, Hayot G, et al. A dominant vimentin variant causes a rare syndrome with premature aging. Eur J Hum Genet. 2020;28(9):1218-1230. doi:10.1038/s41431-020-0583-2
- Belbachir N, Portero V, Al Sayed ZR, et al. RRAD mutation causes electrical and cytoskeletal defects in cardiomyocytes derived from a familial case of Brugada syndrome. Eur Heart J. 2019;40(37):3081-3094. doi:10.1093/eurheartj/ehz308
- Kilens S, Meistermann D, Moreno D, et al. Parallel derivation of isogenic human primed and naive induced pluripotent stem cells. Nat Commun. 2018;9(1):360. Published 2018 Jan 24. doi:10.1038/s41467-017-02107-w

Address
Team
Scientific manager

Reprogramming / differenciation :
Caroline Chariau, TCH 
Margot Hallet, AI 
Elsa Lemaitre, AI
Isabelle Leray, AI 
Yevgeniya Simon, TCH 
Genome Editing :
Sarah Tessier, IE 
Rozenn Le Bloas, IE 
Partners and certifications


Funders